When filtering impurities out of drinking water, mechanical filtration typically is used. But what happens if there are particles in water that are dissolved or are too small and cannot be removed by mechanical filtration?
Fortunately, there is a process called adsorption, which can remove very tiny particles or dissolved contaminants from water such as lead, PCBs some pesticides, viruses and asbestos fibers.
What is Adsorption?
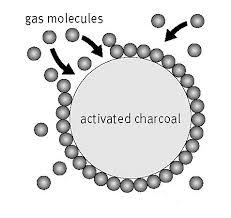
Adsorption is a physical process in which dissolved molecules or small particles (the adsorbate) are attracted and become attached to the surface of something larger (the adsorbent).
The attraction is similar to that of a magnet on a refrigerator, but on an atomic or molecular scale. Energy differences and electrical attractive forces, known as van der Waals forces, cause molecules of the adsorbate to physically fasten and stick onto the adsorbent.
Common in nature, adsorption often occurs between solids and liquids or gases. It is responsible for the transport of nutrients into the soil, assisting in plant and animal growth, the chemical separation of proteins and enzymes, and industrial processes like air purification, sugar refining and desalting of seawater.
Adsorption should not be confused with the completely different process of absorption, in which liquids and particles penetrate into another substance, such as a sponge that soaks in liquids.
Throughout history, people have used carbon (charcoal) as an effective adsorbent, in such processes as water treatment, sugar purification and color removal from liquids. In water treatment systems, an improved form of carbon, called “activated carbon,” is the adsorbent most commonly used to attract and hold dissolved contaminants.
Activated carbon is made from carbon-based materials like coal or wood that is first heated without oxygen to produce charcoal. The charred material is then heated with steam or carbon dioxide to above 1000 degrees Celsius, which further erodes and corrodes it to remove everything but the carbon.
The result is an airy, delicate structure that is nearly pure carbon and full of holes. It is then crushed to a powder and mixed with binders to form granules of desired size ranges for different filter media.
Chemicals are sometimes added during activation of the carbon to produce different surface chemical natures that adsorb different contaminants. For example, acids produce carbon with maximum capacity for adsorbing heavy metals.
Activated carbon’s enormous surface area is a critical factor in its effectiveness to adsorb various contaminants. The surface area typically is about 1,000 square meters per gram. As an example, a piece of carbon the size of a pea has an area the size of half a football field. The structure and distribution of pores in activated carbon are key factors for adsorption because they determine the size of molecules that can be adsorbed.
Depending on the desired results, activated carbon may be used in powdered or granular form. Granular activated carbon is commonly used in water treatment facilities where the water is passed through a granular carbon bed to remove tastes, colors, odors and dissolved organics.
Powdered carbon is the preferred choice in point-of-use water filtration systems because it is a faster and better mechanical filter than granular activated carbon. It also takes up a minimum of space given its large surface area-to-volume ratio.
Water Filter Solutions
Filters with activated carbon are available in a variety of types and sizes, depending on use in the home or commercial operations.
Their effectiveness at adsorption depends mainly on how long the water is in contact with the activated carbon. The longer that water is in contact with the activated carbon, the more materials can be adsorbed.
Point-of-use systems include faucet-mounted, countertop, pour-through and under-the sink filters. The faucet-mounted is the most common filter, which can be easily and quickly installed by attaching it directly to a faucet outlet or on the counter attached with flexible tubing. Under-the-sink activated carbon systems come in two types; ones that filter all cold water passing through the faucet and others that attach to the cold water line and go to a separate faucet, in order to prolong the life of the filter.
Whole-house units filter all water serving a household. These systems are key in removing contaminants that can be absorbed through the skin during bathing or showering, or from inhalation.
For activated carbon filters to be most effective, cartridges need to be replaced periodically. The life of the cartridge varies with the amount of water passing through the filter and the amount of impurities or contaminants present in the water. Expected minimum capacities are expected to be declared on the product labels.
Effect on Water Quality
As activated carbon adsorbs dissolved molecules and sub-micron particles, the effect is the reduction of contaminants resulting in more aesthetically pleasing and healthy drinking water.
Adsorption removes disinfectant chlorine that is often used in municipal water treatment. The taste and odor of disinfectant chlorine is the most common complaint and the most common reason people buy filters.
Adsorption can remove many kinds of pesticides and other synthetic organic chemicals, including chlorinated hydrocarbons, gasoline, industrial solvents and disinfection by-products. Adsorption also can remove heavy metals like lead and cadmium that get in water from corrosion of plumbing materials.
Third Party Certification
While there are many brands of filters on the market, very few of them are third party certified. Certification ensures you that the claims of the manufacturer have been tested and validated.
A trusted and globally recognized certification agency is called the National Sanitation Foundation, or NSF. NSF is a not-for-profit co. founded in 1944 to promote good sanitation. NSF maintains state-of-the-art laboratories where products can be tested according to the set standards. Manufacturers voluntarily submit products for evaluation; if they pass then they are “Listed” and certain tested claims are “Certified” and the products are authorized to display the NSF seal non labels and literature. Although non-governmental, NSF does have some official status, including being the lead agency under contract with the United States Environmental Protection Agency (EPA).
NSF standards are recognized around the world, including the International Standards Organization (ISO) and the Canadian Standards Association (CSA).
Always look for filtration solutions that are NSF certified. In the case of chlorine removal, insist on Standard 42 for Chlorine Reduction, Class I. Anything less than Class I is substandard and not worth the investment.
Closely check the NSF certified capacity rating, shown in US gallons. (e.g. 500 gallons) This rating indicates the number of gallons the system is certified to remove chlorine for. It is not an indication of how much water you will necessarily get. Depending on water quality, water pressure and volume of water consumed, your actual filter life may be more or less. However, the NSF rating assures you that the system can comfortably provide that amount of water chlorine free.